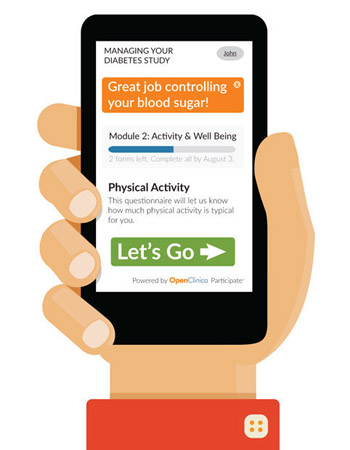
OpenClinica unveiled Participate, a device-independent, mobile solution for engaging participants in clinical research. Participate makes it easier to meaningfully engage patients and collect timely data in a way that is convenient for patients.
Using Participate with OpenClinica EDC allows researchers to design participant-facing events, reminders, and assessments alongside clinical visits and CRFs, using OpenClinica’s existing, user-friendly study build tools. All the clinical and patient reported outcomes data are seamlessly captured in a single place, and participants’ activities become part of the same regulatory compliant audit trail that tracks all clinical user activity. The data participants submit are immediately available for review and analysis alongside CRF data from other sources.
In comparison, the traditional study methods involve relying on antiquated paper diaries or the provisioning of additional devices to patients, both of which are expensive, logistically complex, and unreliable.
“We live in a world where 90% of adults have smartphones, 81% text, and 63% use their phone to go online. We need to reach patients on their terms, whether email, SMS, web, or a mobile device,” said Cal Collins, OpenClinica’s CEO. “Treating research volunteers as participants, as opposed to subjects, can lead to concrete benefits, such as improved compliance more effective recruiting, and more complete, timely data. It can even help enable new protocol designs that better target populations and/or more closely align with real-world use. But most of all, it just seems like the right thing to do.”