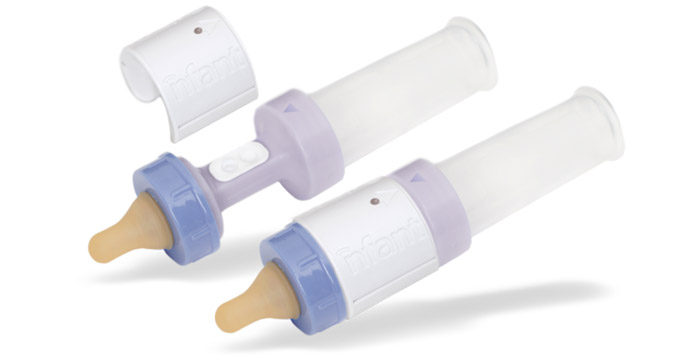
NFANT Labs‘ has received FDA clearance for its smart infant-feeding solution, which monitors tongue movements during feeding to provide objective, measurable data.
The company claims its product is the only medical device on the market that relays information to caregivers through a mobile app and is stored in the cloud. Competing offerings are not “medical grade” solutions.
NFANT Labs plans to begin offering the nfant Feeding Solution to NICUs later this quarter.“Since beginning product development just a year and a half ago, we have successfully crossed another hurdle by receiving our FDA clearance,” said Lou Malice, CEO of NFANT Labs, in a statement. “Up to 70 percent of the 540,000 premature babies born in the U.S. each year experience feeding problems. Until now, physicians and caregivers have not had access to objective feeding data and the power of cloud analytics to enhance decision making.”
At the moment, feeding decisions are made based on a caregiver’s experience and trial-and-error, but now nurses and physicians will be able to use the device’s data to determine when infants are ready to transition from tube feeding to bottle or breastfeeding.
NFANT Labs plans to begin offering the nfant Feeding Solution to neonatal intensive care units (NICUs) later this quarter, with the idea to expand to other markets next year.
[Via: mobihealthnews, bizjournals]