Janssen Research & Development has established the Integrated Smart Trial & Engagement Platform (iSTEP), touting it as a first-of-its-kind information technology toolset developed to automate investigational product supply and data management in clinical trials. In other words, the system is designed to replace the paper-based conventional processes of managing clinical supplies and tracking patient health data with a “cohesive suite of digital technologies.”
The iSTEP platform leverages mobile technology, “smart” packaging and electronic labels in an effort to streamline and automate the investigational product management process in clinical trials. The system integrates smartphone apps to support patients in following their treatment schedule and interacting with their healthcare provider while participating in a clinical trial.
“As clinical trials grow in complexity, duration and cost, we are adopting different technologies to optimize workflow, improve communication, and expedite data reporting, all critical success factors in clinical trials,” Andreas Koester, M.D., Ph.D., Vice President, R&D Operations Innovation at Janssen, said in a statement. “The open innovation philosophy at Janssen led us to develop iSTEP in a way that allows the technology to be available to other pharmaceutical companies. We believe that having a consistent approach across the industry can accelerate the process of bringing medicines to patients.”
The iSTEP platform consists of four modular components:
1. eTracking
Uses scanners to verify and register all medication kit activities at the study site, including receipt, dispense and return of kits. The design allows information to become immediately available to physicians and the study sponsor via a web portal, in an effort to eliminate paper documents and manual data entry, and to reduce dispensing errors.
2. eCommunication
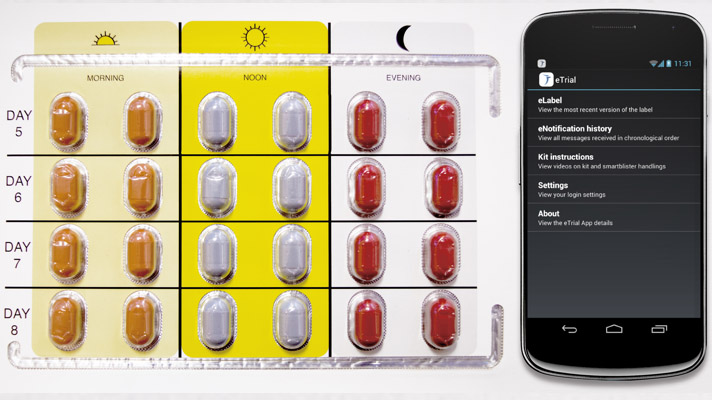
This module provides information that can be tailored to patient needs, such as reminders, dosing instructions and tutorial videos via their smartphone to encourage medication adherence. It also is designed to collect certain data, such as adverse events.
3. eLabel
It is designed to augment complex, multi-language booklets that are customarily distributed in clinical trials, with an electronic drug label, supplemented with other patient-specific data, in the patient’s native language. eLabel’s flexibility is conducive to adaptive trial designs, as updates to the protocol can be made after the trial has been initiated and efficiently communicated to patients.
4. eAdherence
Includes smart medication blister packs that register each pill as it is removed, to help track medication adherence. Continuous monitoring of medication adherence may enable real-time intervention.
Janssen has already conducted a technical pilot with iSTEP and is working with health authorities and ethics committees to implement a pilot assessment of iSTEP within a clinical trial by the end of 2017. Going forward, Janssen plans to progressively implement iSTEP into a variety of clinical trials in its portfolio.
Janssen collaborated with Tata Consultancy Services (TCS), to build-out iSTEP as part of TCS’ Connected Clinical Trials (CCT) platform. Companies interested in obtaining access to the platform can contact TCS directly.