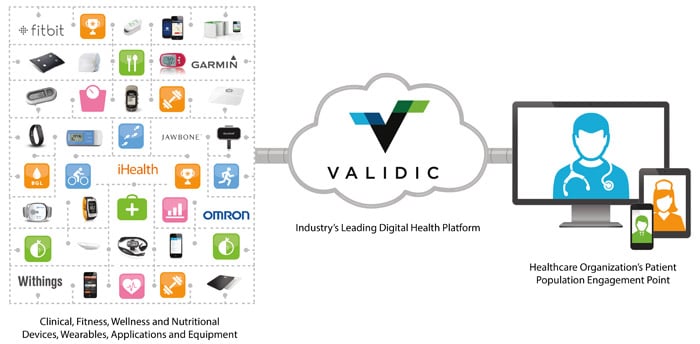
Medidata and Validic are teaming-up to “propel connectivity and innovation” in clinical research by expanding access to patient-generated data from a range of consumer and medical grade mHealth devices and apps. By integrating Validic’s digital health platform with the Medidata Clinical Cloud, the two parties are providing the technology infrastructure needed to transform clinical trials through the use of patient-generated data that is secure, regulatory-compliant and actionable.
As a result of this collaboration, trial sponsors will be able to gather data from more than 175 devices and apps in the Validic ecosystem.As a result of this collaboration, trial sponsors will be able to gather data from more than 175 devices and apps in the Validic ecosystem — including those from Garmin, Fitbit, Jawbone, Withings and many others — and integrate it into the Medidata platform. Once data is transferred through the secure, regulatory-compliant environments of Validic’s platform and the Medidata Clinical Cloud, patient data from the mHealth tools is then mapped to the clinical record and unified with traditional clinical measures.
“Our partnership with Validic is a big step toward realizing the potential of mobile health in clinical research because it offers life sciences organizations the flexibility to select the mHealth tools that provide the most clinically meaningful information for specific patient populations,” said Glen de Vries, Medidata’s president.
Validic’s FDA Class I Medical Device Data System (MDDS) delivers standardized and HIPAA-compliant patient health data from a number of different consumer and medical grade devices and apps. On the other hand, Medidata’s cloud-based infrastructure gathers patient data from these mHealth tools and integrates it with other traditional data collected in studies, including lab information, clinician-entered vital signs and adverse events. The Medidata platform is currently enabling top life sciences companies to conduct Phase I-IV mHealth clinical trials worldwide.